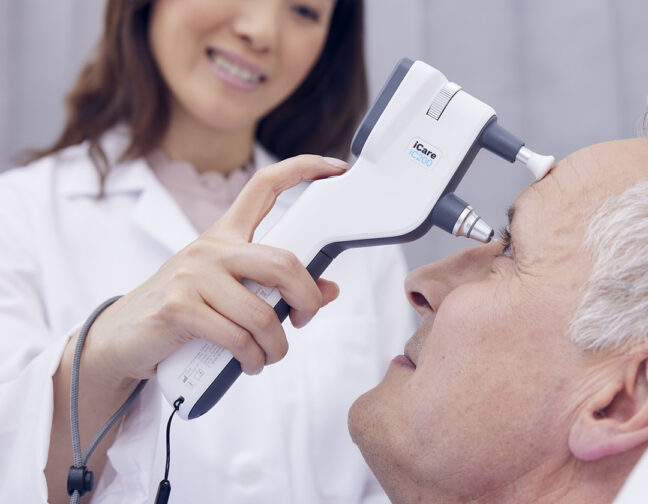
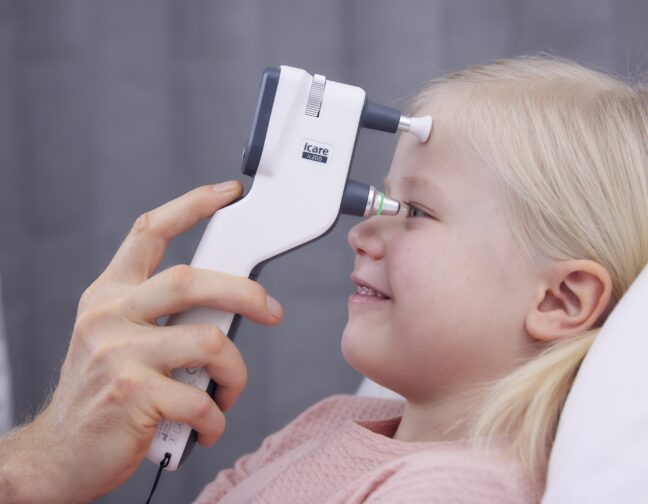
iCare, the original developer of rebound tonometry, has transformed tonometry by delivering innovative, easy-to-use tonometers for both professionals and patients. This dedication to excellence extends to iCare probes, which are integral accessories to our tonometers.
iCare probes are specially designed to ensure safe, hygienic, and accurate IOP measurements. Below are five reasons why you should always use the original, certified iCare probes.
1. The accuracy and repeatability of iCare tonometers are validated only with iCare probes
The probes are integral to accurate functioning of iCare tonometers. They are subject to a rigorous design and development process to ensure that every IOP measurement is accurate and reliable. The probes maintain stringent weight, length, and straightness tolerances to ensure consistent IOP measurements.
The manufacturing and usage of medical devices are highly regulated — and their safety and effectiveness need to be validated. Only genuine iCare probes should be used with our tonometers as they work together as a system. The accuracy and repeatability of iCare tonometers are validated only with genuine iCare probes.
2. Optimized design ensures efficient, accurate, and painless measurements
iCare probes are exclusively designed to be compatible with iCare tonometers. They are compact, lightweight, and have a gold-plated metal wire with a small medical-grade plastic tip. The manufacturing process and quality controls of the metal wire and the tip are validated to produce a smooth surface for guaranteed contact with the cornea every time.
During the IOP measurement, the probe makes momentary contact with the cornea in six rapid movements. This allows faster IOP acquisition, especially with challenging patients. In all cases, the process is painless, and the patient experiences minimal awareness of the probe contact.
iCare probes are simple to use — they can be easily inserted and removed from the probe base. The tonometer automatically magnetizes and secures the probe in place.
3. iCare probes are manufactured in a controlled, clean room environment
iCare probes are manufactured in a clean and controlled environment employing conditions of class 7 according to ISO 14644-01 and bioburden control of the environment (EU cGMP Annex 1; USP Chapter 116).
Inspection and routine checks ensure that the hygiene and air cleanliness requirements defined in the above-mentioned standards and regulations are always met. This process guarantees the safety and cleanliness of the probes used in your clinical practice.
4. Hygienic single-use design minimizes the risk of cross-contamination
Patient safety is paramount, and that’s why our probes come individually packed in protective tubes, designed for single use. This minimizes the risk of microbiological cross-contamination and potential transmission of infectious diseases.
Efforts to clean the probe may result in the bending of the metal wire or damage to the plastic tip. This mishandling could impact the reliability of the IOP measurements or harm the device, reducing its lifespan.
5. iCare probes are backed by 20 years of experience in eye care innovation
iCare produces accurate and easy-to-use tonometers that have transformed the eyecare industry for two decades. The reliability and reproducibility of the measurements have been proven in over 200 clinical studies leading iCare to become the market leader in handheld tonometry. This extensive experience has guided continuous innovative refinement of the technology and improved the manufacturing process of the devices.
By choosing iCare, you are benefiting from the accumulated knowledge, research, and innovation of the pioneers in the industry. iCare tonometers deliver the highest standard of care for your patients.
Using the original, certified probes supplied by the manufacturer ensures patient safety, error-free tonometer operation, and efficiency. Using any other probe may damage the tonometer, lead to operational errors, and produce inaccurate results. The use of probes that are not made by iCare voids the manufacturer from all responsibilities and liabilities concerning the tonometer’s safety and effectiveness.