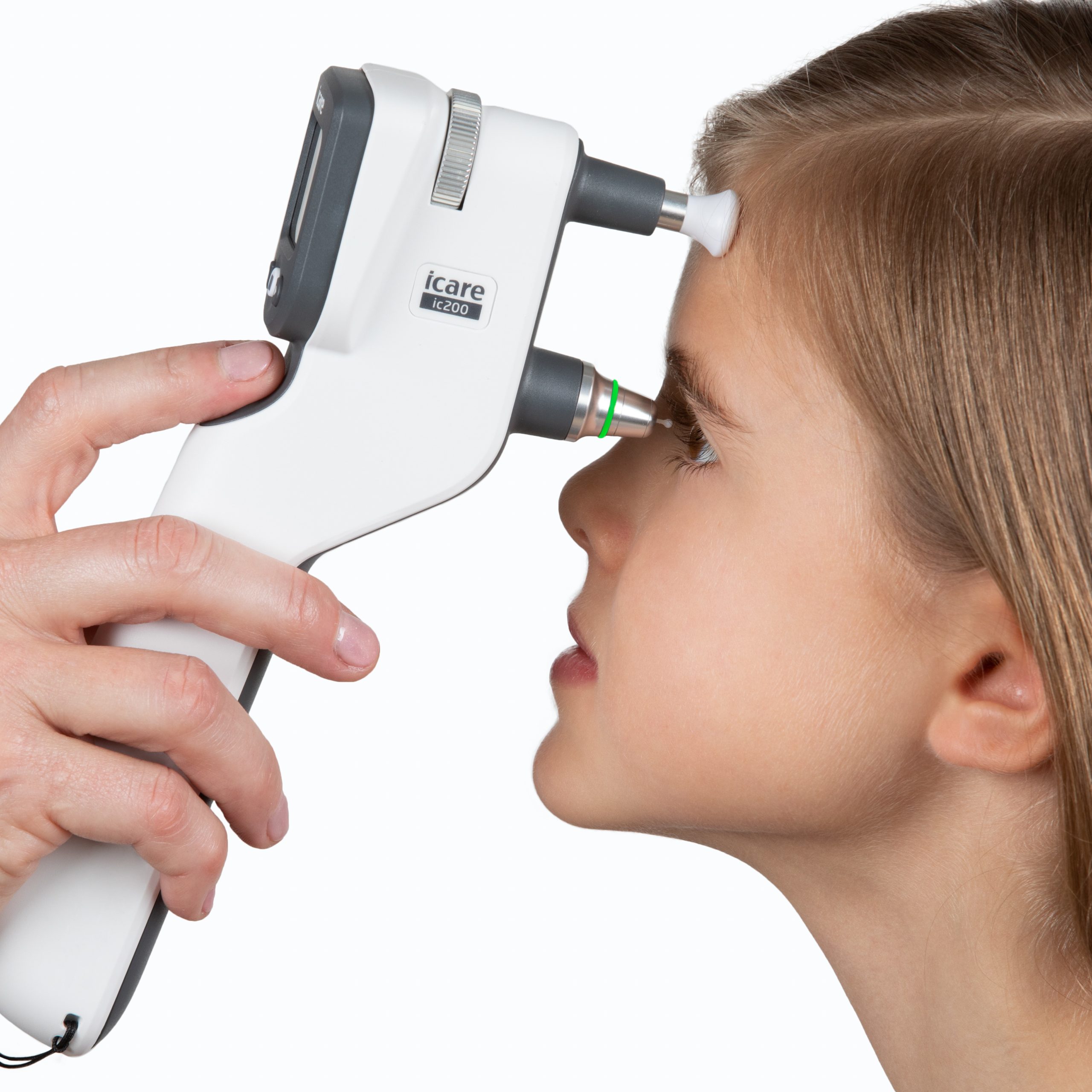
iCare IC200, the new generation tonometer for intraocular pressure screening for professional use has been cleared by FDA, the Food and Drug Administration of the United States. The product has been previously authorized in both Europe and Japan. The FDA clearance now enables the launch of sales and marketing measures also in the United States.